South Korea
Also, AIRS Medical has proved the capability of its AI MRI reconstruction tool to denoise and improve the quality of 3D MR images.
Its AI imaging solutions are coming soon to CARPL.ai's platform.
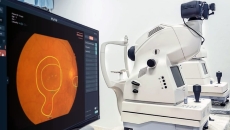
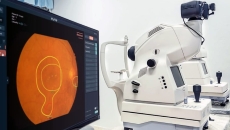
Also, Edenlux is now providing AI-generated eye health reports in its mobile app.
Sonde is its second global digital health investment to date.
Also, Syntekabio has launched its cloud service platform for AI drug discovery.
Also, Max Life in India has developed an analytics solution that detects inaccurate medical reports in real time.
Also, Point Robotics from Taiwan has received a US FDA 510(k) clearance for its surgical robot system.
Individuals are also less likely to trust AI-suggested interventions than those recommended by humans.
The company will maintain its patent right for the technology.
Also, Lunit has received Taiwan's approval for its AI mammography solution.